We wondered if it was possible to simply reverse the reaction of burning oxygen against carbon (coal). We knew it would not be easy and require energy, as the energy released by turning O2 and C into CO2 is considerable.
To get to CO from CO2 seemd the first step, we have not come across a way to directly tear the O2 of CO2. One option is to split CO2 using a cathalyst in an electrochemical cell. Another option is a reaction that turns CO2+H2O into CO and H2. This process can be done at high temperatures, but there are other ways as well.

So after some process that requires either electric or thermal energy we end up with a lot of CO, this was where our search based on our knowledge ended. Now we find there is a reaction that is the considered in a lot of CO2 generating installations, that shows how you can make CO2 and C from CO, a reaction named after Octave Leopold Boudouard, The Boudouard reaction. It is quite simple:
2CO ⇌ CO2 + C
So starting with two CO molecules you can one CO rip the O of the other leaving it as C (black carbon soot). It is a reversible reaction, where the temperature determines the side that “wins”. The lower the temperature the more likely carbon is formed.
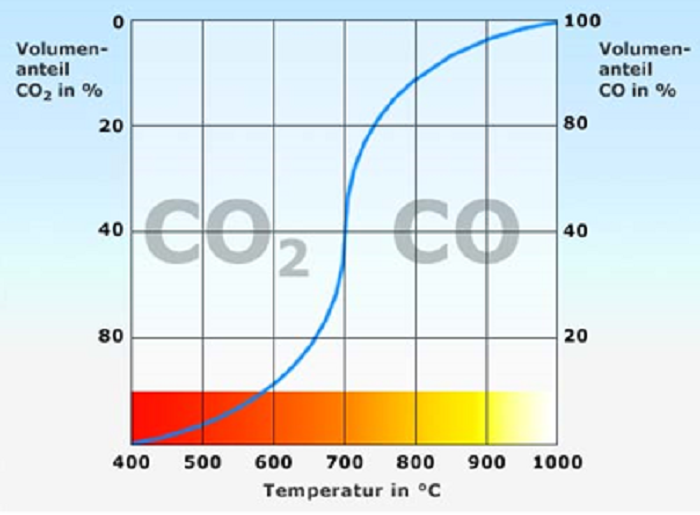
Many coal or gas burning installations produce CO, and this CO can turn into C and CO2, and because C (Carbon) can clog up any cleaning processes (cathalysts) downstream, it is normal to keep the temperature of these gasses high, so it remains mostly CO. Industry is expending energy to prevent the production of Carbon. We could ask them to filter out the C instead, and they would need less input or could help test carbon sequestration processes.
So there is an option to turn CO2 into pure carbon C. The process would generate CO gas out of CO2 and H2 and then lead this gas into a space with the right temperatures to generate the Carbon. If you wanted to sequester the Carbon for good, you could make it so it can precipitate down in the space filled with CO to form a layer on the ground. If you could constantly extract the CO2 and replace it with CO you could have a continuous process to draw down carbon.
The worlds coal reserves where formed in a time when trees just entered the scene, the bacteria in those days could not digest an important constituent that gave the trees their strength, lignin. It took a long time before they managed to do it, and all the trees in the mean time took CO2 and H2O out of the atmosphere, cooling down our planet. All this lignin became the coal we burned for the last two centuries. We need to put at least the carbon back into the ground.
We would like to see reactors for the above process. Ones that you put CO2 and H2O in on one side, and get C and CO2 (and H2 by the way) out on the other. once you have such a reactor you can scale up power plants to generate layers of carbon soot in the ground. You could sink it to the ocean floor, powering the process with wave energy or burry it in mines. This would be a process able to stand high temperatures, which will dominate large part of the planet soon. Land not being used by living things because it is too hot would be available to build the necessary installations.